[
{
"name": "Top Stories Video Pair",
"insertPoint": "7",
"component": "17087298",
"parentWrapperClass": "fdn-ads-inline-content-block",
"requiredCountToDisplay": "1"
}
]
Nothing threatens our future more than the existence of nuclear weapons, as documented by a free DVD you can order from the Web site Nuclear Tipping Point (www.nucleartippingpoint.org). And don't be lulled by our agreement with Russia to reduce mutual stockpiles to 1,550 warheads. Can you even name 1,550 U.S. cities? Two Japanese cities have already seen nuclear devastation -- a subject fellow Journal scribe Barry Evans has explored ("Hiroshima: 64 Years On," Aug 6, 2009).
Would terrorists hesitate to use nuclear weapons if they obtained any?
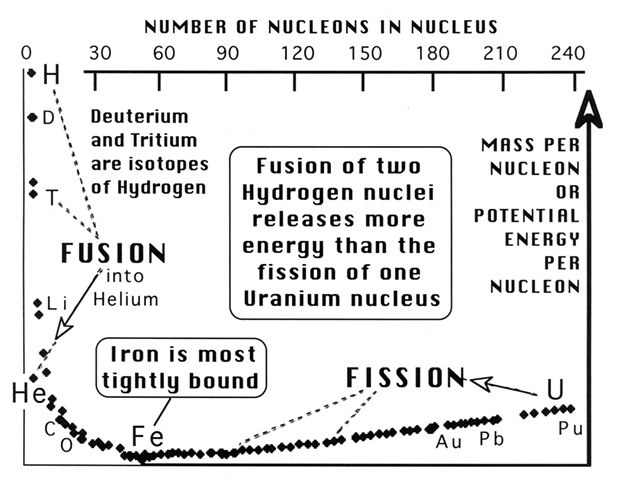
Here is the science behind the threat: A speck of dust may contain a trillion atoms, yet an atom is huge compared to its nucleus composed of nucleons (protons & neutrons) bound together by gluons. This binding even overpowers the electrical repulsion between crowded protons. According to Einstein's E = mc², the potential energy per nucleon is proportional to the excess mass per nucleon relative to that in iron, the most tightly bound nucleus, as shown in the diagram.
Nuclear weapons release energy by fissioning apart large nuclei (uranium or plutonium), or fusing together small nuclei (hydrogen isotopes derived from lithium deuteride). The Nagasaki bomb converted 1,000 grams of plutonium into 999 grams of radioactive fission products plus 1 gram of energy. The largest fusion bomb tested by Russia yielded 2,300 grams of energy! E = mc² is universal: 50 Megatons of TNT would also yield 2,300 grams of energy. Fission has been controlled to generate electricity, but controlling fusion remains a dream.
How did the universe provide us with sources of energy? Well, hydrogen and some helium came directly from the Big Bang, but the heavier nuclei were forged in stars. Stars initially fuse hydrogen into helium, as does our Sun. Red Giants then fuse helium into carbon. The end product of stellar fusion is iron (and nickel) at the bottom of the potential energy curve. Nuclei heavier than iron require an input of energy. That energy is derived from the gravitational collapse of the iron cores of massive stars, which result in supernovae explosions. These not only produce 66 elements beyond iron, they crucially scatter all 92 back into space where they can eventually accumulate into new stars, planets and fragile humans.
Don Garlick is a retired professor of geology.