[
{
"name": "Top Stories Video Pair",
"insertPoint": "7",
"component": "17087298",
"parentWrapperClass": "fdn-ads-inline-content-block",
"requiredCountToDisplay": "1"
}
]
Our comfortable levels of atmospheric oxygen (O2) and carbon dioxide (CO2 ) are attributable to photosynthesis: H2O + CO2 = CH2O + O2. It is thus reasonable to assume that forests are needed to preserve the oxygen we breathe. But that is a common misconception. A mature redwood or Amazon forest busily recycles its products, yielding no net oxygen. Only if the wood is protected from rotting, as in a swamp, will net oxygen be released instead of recombining with dead wood.
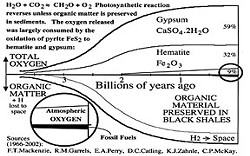
Photosynthesis reverses unless organic product is protected from oxygen. Fortunately, over the past 3 billion years, a small fraction of organic matter production was sequestered into shaley sediments including fossil fuels. However, the first billion years of released oxygen was entirely consumed by the oxidation of pyrite (FeS2) and other minerals into gypsum and hematite (see diagram). Only after these demands were somewhat satisfied, 2 billion years ago, did atmospheric oxygen begin to accumulate. Currently, gypsum stores 59 percent of net oxygen, hematite stores 32 percent and the atmosphere stores 9 percent. Our vast deposits of gypsum and iron ores are products of photosynthesis!
Combustion of all the world's forests would consume a negligible fraction of the atmosphere's oxygen. On the other hand, the facts presented above imply that we would consume all our free oxygen by burning less than 10 percent of the organic material preserved in sediments. Luckily, the proportion of that organic sediment that is feasibly extractable as fossil fuel (mostly coal) is so small that the oxygen content of the atmosphere would remain above 20 percent (currently 20.9 percent). The dire consequences of converting fuel into carbon dioxide is another story (next week).
We must conserve our forests for many reasons, including their carbon content. But one of the few resources we need not worry about is oxygen, thanks to black shale.
The geologically-correct photosynthetic reaction is:
218H20 + 4CO2 + 48CaCO3 + 70FeCO3 + 24FeS2 + 14FeSiO3 = 122CH2O + 48CaSO4 + 2H2O + 54Fe2O3 + 14SiO2 + 11O2
Don Garlick is a geology professor retired from HSU. He invites any questions relating to North Coast science, and if he cannot answer it he will find an expert who can. E-mail [email protected].
Speaking of Science
-

HSU Expanding Curriculum with Polytechnic Push
Jun 15, 2021 -

Davos Won't Save Us
Jan 23, 2020 -

The Need to Study Weed
Jan 16, 2020 - More »
more from the author
-
Nuclear Matters
- May 6, 2010
-
Sophie Smells a Shaker
- Feb 4, 2010
-
The Roots of Love
- Feb 5, 2009
- More »